What Will Happen If You Put Copper In A Fast Plant
Introduction
Heavy metal like cobalt (Co), copper (Cu), iron (Atomic number 26), manganese (Mn), molybdenum (Mo), nickel (Ni), and zinc (Zn) enters in soil from various sources such as mining, foundries, smelters, combustion, and agriculture (Nagajyoti et al., 2010). Establish genomes encode a number of transporters that are specific in their substrate specificities, expression, and in cellular localization to manage the translocation of these metals into the whole plant (Colangelo and Guerinot, 2006; Hwang et al., 2016). These metals are interim a beneficial role for plant growth, evolution, and productivity at an optimum concentration in the form of the essential micronutrient (Singh et al., 2016). To grow and complete the life cycle plants use these essential micronutrients (Wuana and Okieimen, 2011). The plant takes these essential heavy metals like iron, zinc, copper, and manganese from the soil due to concentration gradients and selective uptake of these metals (Peralta-Videa et al., 2009). These ions enthusiastically affected the part of many enzymes and cellular metabolism. These metals also play a prominent role in the synthesis of protein, nucleic acids, photosynthetic pigment, and it too take part in the structural and functional integrity of cell membranes (Oves et al., 2016). For instance Copper is an essential heavy metal which actively takes part in the photosynthesis (Gad, 2012). Manganese is an important elective of various metabolic enzyme similar mallic dehydrogenase and oxalosuccinic decarboxylase (Millaleo et al., 2010), Cobalt constitute in the form of Vitmain B12 (Barker and Pilbeam, 2015), while Fe act as a cofactor in cytochrome (Thomine and Lanquar, 2011). Although the concentration of these heavy metal ions in soils is severely altered past the arbitrary human activities and through the various natural procedure (Singh et al., 2011). The enhanced concentration of these benign ions poses a toxic effect into the found cells. These furnishings may be substituted of essential functional groups, cellular damage, generation of reactive oxygen species (ROS), disturbance in the various metabolic reaction by altering the enzymatic activity (Anjum et al., 2015). Regarding the above facts, it is noticeable that only a express amount of these beneficial heavy metals is essential for the constitute growth and metabolic function. Therefore, in the electric current article we elaborately reviewed various studies regarding heavy metals sources, their uptake mechanism, essential transporters and also discuss near the effective, and subversive properties of heavy metals in response to their concentration.
Source of Various Beneficial Heavy Metallic Ions
At that place are numerous source of HMs contamination in the environment like natural, and anthropogenic including agricultural, industrial, domestic, and atmospheric (Bing et al., 2011). The most imperative natural source of HMs contamination is geological bedrock and rock substratum (Tchounwou et al., 2012). The limerick and amount of heavy metal specifically relies on the type and concentration of rocks and as well as on the weathering process (Wuana and Okieimen, 2011). The inorganic and organic fertilizers are the agronomical sources of heavy metal contagion, liming, sewage disposal, irrigation water, and pesticides are the principal cause of heavy metal discharge in the soil (Chopra et al., 2009). A example study around peri-urban and urban-industrial clusters in Ghaziabad, India, reveals that waste product water irrigation is responsible for the heavy load of heavy metallic in agronomical soils, crops, and vegetables (Chabukdhara et al., 2016). Mining refinement such as spoil heaps, tailings, transportation of ores, smelting metal finishing, and recycling of metals are the industrial procedure that liberates the HMs in the surroundings (Tchounwou et al., 2012), For instance, Deng et al. (2016) through their study suggested that the atmospheric deposition is the major crusade of Pb, Cd, Cu, Cr, and Zn accumulation in plants of peri-urban and smelting contaminated sites in Baoji, China. While the explosion, landfills, and transportation like automobiles, diesel powered vehicles, and aircraft are also the source of heavy metal pollution (Wuana and Okieimen, 2011). Anthropogenic activities like coal mining, waste product combustion, and steel processing are the major cause of rising level of zinc (Lottermoser, 2010). The excessive injudicious and unregulated apply of Cu fungicides, bactericides and Chromium (Cr) contaminates the environment through the electroplating processes and waste pesticides to control institute diseases and pest that has resulted in Cu accumulation in surface layer of agricultural soil (Mackie et al., 2012).
Uptake and Translocation of Benign Heavy Metal Ions
Soil is the reservoir of various HMs contaminations and has strong property of cation substitution chapters. Amid these HMs some of the metals such as Co, Cu, Cr, Atomic number 26, Mg, Mn, Mo, Ni, Se, and Zn are essential element, that are required in very small amounts for optimum found growth and development (Alloway, 2013). These benign HMs plays several biochemical and physiological task in plants and as well regarded as significant constituents of various cellular enzymes moreover actively take part in several oxidation-reduction reactions (Emamverdian et al., 2015). For instance, Fe easily reduced and oxidized in various biochemical processes and also an important cofactor of many enzymes which involves in the respiration, photosynthesis, and nitrogen assimilation (Hell and Stephan, 2003). Zn is a vital structural elective of poly peptide and also acts as a cofactor of several enzymes (McCall et al., 2000). Zn absorption, uptake, and accumulation in plants occurs throught the involvement of Zinc transporters and metal chelatiors into the constitute (Gupta et al., 2016). Cu also acts equally an essential element for plant growth by participating in many redox-agile reactions. Mn plays an of import role in detoxification of ROS (Ducic and Polle, 2005). Plant blot essential and not-essential element from the soil in response to concentration slope and selective uptake of ions or by improvidence (Peralta-Videa et al., 2009). The absorption level of unlike element relies upon the different plant species. Root plays a significant role in the agile uptake of metal ions. The mechanism is mainly started by the absorption of metal ions in the root tissue, the ions of Co, Cu, Iron, Mn, Mo, Ni, and Zn dissociates from its complex forms at the root surface. The metals are heavily accumulated into the root apoplast (Krzesłowska, 2011). The adsorption of heavy metals on the root surface takes place in cationic grade with negative prison cell wall due to the presence of cellulose, pectins, and glycoproteins that work as specific ion exchangers. The adsorption and translocation of metal ions occurs in xylem and phloem tissue through the root past two means known as apo-plastic and symplastic (Hossain et al., 2012). The apoplastic transportation occur through the intercellular spaces by the improvidence of metal ions in the root cell through the soil solution, while the symplastic transportation of metal ions takes place through the plasma membrane past the dissimilar carrier or transporters (Barberon and Geldner, 2014).
Metallic Transporters
Beneficial metal food elements like Co, Fe, Mn, Cu, Mo, Ni, and Zn are essential for normal institute growth and development (Loftleidir, 2005). These metal nutrients occur in the soil in limited corporeality and transported to the plant in a homeostatic fashion by the metal transporters (Krämer et al., 2007). Several workers disclose the part of transporters in beneficial metal adsorption and translocation in plants (Krämer et al., 2007; Puig et al., 2007). Grotz and Guerinot (2006) reported the uptake of Iron and Zn ion which is mediated by a group of transporters belonging to the Cypher family like ZRT (Zinc regulated transporters) and IRT (Fe regulated transporters) proteins in higher plant. Kim and Guerinot (2007) stated in his commodity that IRT1 is able to mediate the transfer of multiple metals including Fe, Mn, Zn, and Cd. Hussain et al. (2004) in their article "P-Type ATPase heavy metal transporters with roles in essential zinc homeostasis in Arabidopsis" reported the role of HMA2 (Heavy metal ATPase) and HMA4 in essential Zn homeostasis (Table S1). The assimilation and translocation of copper occurs in plants by the CTR (Copper transporter) and COPT1 (Copper transport protein; Sancenón et al., 2003). Cu transported in plants past the 2 types of transporters first ane is P-type ATPases belonging to the HMA family and 2d i is RAN1 (Responsive-to-Antagonist) as well known as HMA7 (Sancenón et al., 2003; Tabular array S1). Colangelo and Guerinot (2006) stated in their article that YSL (Yellow strip-like) members accept been involved in the transportion of metals such as Fe and Mn ions in rice plants (Table S1). Mizuno et al. (2005) reported the three Aught/NRAMP (natural resistance-associated macrophage poly peptide) transporter genes from a Ni hyperaccummulator plant Thalpsi japonicum and their Ni–ship abilities (Table S1). The obtained result suggested that Cypher/NRAMP transporter contributes in Ni homeostasis in plants (Table S1). TjZNT1 has Zn, Cd and Mn ion transportation power and TjZNT2 besides has Zn and Mn transporting chapters, while TjNRAMP4 could only transport Ni (Table S1).
Impact of Benificial Heavy Metals on Plants at Low Level
Beneficial HMs like Co, Cu, Atomic number 26, Mn, Mo, Ni, and Zn (Blaylock and Huang, 2000) at low level or under an optimum range induces essential biochemical and physiological reactions in plants (Nagajyoti et al., 2010). Cobalt plays an essential part in plant growth development past regulating establish water utilization and reducing transpiration rate (DalCorso et al., 2014). Gad and Hassan (2013) carried out an experiment on tomato found with Co application at 7.five ppm, which enhanced the growth, yield, food levels, and chemical constituents of lycopersicon esculentum plant with improve quality of fruits (Table i; Figure i). Copper being an essential HM, at low amount helps in enhancing the plant photosynthesis (Mahmood and Islam, 2006). It involves in physiological functions and is a crucial cofactor for many metaloprotiens (Yruela, 2005). Copper is a vital chemical element for plant growth and development (Table i; Figure 1), likewise proved as a micronutrient for plants (Kabir et al., 2009) and it plays an imperative function in CO2 assimilation and ATP synthesis (Pichhode and Nikhil, 2015). Cu at optimum level is valuable element of diverse proteins such as plastocyanin of photosynthetic system and cytochrome oxidase of respiratory electron send chain in plants (Demirevska-Kepova et al., 2004). Because Fe every bit beneficial HM for plants, it is essential for respiration, photosynthesis, nitrogen fixation (Table 1; Effigy i), various cellular processes similar DNA synthesis and hormone production (Becana et al., 1998; Møller et al., 2007), chloroplast development and chlorophyll biosynthesis (Møller et al., 2007). Information technology is a constituent of heme protein (cytochromes, catalase, peroxidase, and leghemoglobin) and iron sulfur poly peptide (ferredoxin, acontiase, and SOD; Gill, 2014). Low pH level of soil makes the Fe more readily available for the plant root Fe uptake (Marschner, 1995; Asati et al., 2016). Manganese is a vital plant nutrient element; predominantly it plays an imperative function in structuring photosynthetic proteins and enzymes and positively affects the biosynthesis of growth substances (Table 1; Figure 1) and the gene expression (Frassinetti et al., 2006). It likewise regulates the metabolism of carbohydrates and lipids, relocation of trace ions, and other HMs in soils (Marschner and Rengel, 2007). Shenker et al. (2004) propounded about the Mn nutritious possessions on lycopersicon esculentum (Lycopersicon esculentum) and role of Mn on enhancing growth, chlorophyll content and SOD (superoxide dismutase) activeness of lycopersicon esculentum plant at vii.6 and eight.6 mg kg−i Mn concentration (Table 1; Effigy i). In meaning perspective of Mo, it is a constituent of more than than 60 metalloenzymes and proteins (Kaiser et al., 2005; Mendel and Schwarz, 2011). Plant requires Mo in the range of 0.i–ane.0 ppm (McGrath et al., 2010), enhances the total chlorophyll concentration in plants (Datta et al., 2011). Nickel is another beneficial chemical element for plants. It required past the plant in a very petty amount for normal plant growth and functioning (Izosimova, 2005). Zinc is a crucial chemical element that influences a number of metabolic processes of plants, it also plays a significant role in producing chlorophyll thus it is vital for normal plant growth (Table 1; Figure 1). Even so beneficial HM ions like, Co, Cu, Fe, Mn, Mo, Ni, and Zn likewise regulate plant's ROS scavenging system involving enzymatic and non-enzymatic antioxidants mechanisms (Gill and Tuteja, 2010).

Table 1. Upshot of beneficial heavy metallic on different plants at depression level.
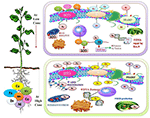
Figure 1. Beneficial heavy metals are play a pregnant role at low concentration in the synthesis of protein, nucleic acids, photosynthetic paint, and it also involved in the structural and functional integrity of jail cell membranes (Oves et al., 2016). Mn promotes antioxidant activity (Shenker et al., 2004), Fe promotes Due north2-Fixation and DNA repair (Møller et al., 2007). While at high fconcentration it crusade substitution of many essential functional groups, for example, lipid peroxidation (LPO), cellular damage, generation of reactive oxygen species (ROS), disturbance in the various metabolic reaction by altering the enzymatic action (de Oliveira Jucoski et al., 2013; Anjum et al., 2015).
Impact of Benificial Heavy Metals on Plants at Loftier Level
Plants are frequently sensitive both to the low and high accessibility of some heavy metallic ions as essential micronutrient. Beneficial heavy metals at high level could upsets the soil environs that consecutively adversely influences soil fertility, plant growth and evolution (Reeves and Bakery, 2000). The threshold level at 10 ppm for Ni and fifty ppm for Co has been proved undamaging for chickpea (Table 2; Effigy 1). Ni at more than than l μg Ni yard−1 dry weight negatively affects growth of the plants at multiple levels such as morphological, physiological, and biochemical (Khan et al., 2006). Bakkaus et al. (2005) quoted average Co concentration for plants betwixt 0.1 and x μg g−one dry weights and also elaborated the benign office of Co for the normal metabolic operation of plant at low concentration (Tabular array 2; Figure 1). Whereas, several studies has been already proved the toxic effects of Co at higher concentration that get toxic for the normal plant growth and development and at the same time alters several processes inside found cell (Parmar and Chanda, 2005; Jayakumar and Vijayarengan, 2006; Jayakumar et al., 2008; Khan et al., 2006; Khan and Khan, 2010). Jayakumar et al. (2008) reported the improved seed germination and increased length of radical and plumule of ragi and paddy at low dose of Co (v μg L−1) while reverse condition was observed at high dosage (25–100 μg Co L−one). Cu in backlog hinders plant growth and disables cellular processes such as photosynthesis electron send (Yruela, 2005). Li et al. (2009) studied toxicity of Co on barley (Hordeum vulgare L.), oilseed rape (Brassica napus 50.), love apple (Lycopersicon esculentum L.), and found out that Co has reduced the shoot growth and biomass of the plant (Table 2; Effigy 1). Khan and Khan (2010) conducted an experiment on chickpea (Cicer arietinum) to evaluate the upshot of nickel and cobalt at lower (0, 10, 50 ppm) and college (100, 200, and 400 ppm) concentrations, obtained result indicated that at loftier concentration Ni and Co induced toxicity in chickpea plant by reducing its growth and biomass, seed formation, chlorophyll content, acquired shoot, and root injury, leaf chlorosis, suppression of root nodules and finally it adversely affects the yield of the plant (Table 2; Figure 1). Cu when nowadays in soil in loftier corporeality causes cytotoxic injury to plants this resulted in a hindrance of plant growth and caused chlorosis (Lewis et al., 2001). Copper toxicity adversely influence the growth, dry out matter, and yield of Vigna radiata (Manivasagaperumal et al., 2011) and growth and oxidative mechanism of tea plant (Camellia sinensis; Dey et al., 2015). De Dorlodot et al. (2005) when plant is subjected to increased Fe2+ uptake and translocation by the establish, Fe toxicity appears (Table two; Figure one). Moreover, Arora et al. (2002) stated near the elevated Fe2+ level in found induces the product of free radicals that causes membrane, DNA and proteins damages (Table two; Figure one). Wu, 2016) too studied fe toxicity in rice plant. Mn at higher level go toxic for plant induces several injuries such as the arrest of establish metabolic processes and the distortion in photosynthetic machinery (Ducic and Polle, 2005). Mn was also reported to inhibit root growth in soyabean (Chen et al., 2016). In excess Mn halt plant growth and evolution by causing interveinal and marginal chlorosis, necrosis, and distorted leafage structure both externally and internally (Kitao et al., 2001), also by reducing photosynthetic charge per unit of the plant, CO2 assimilation and stomatal conductance (Li et al., 2010). The level of iii.0 ppm of Mo on exposed metaltolerant hydrophyte, Trapa natans reported to cause baloney of mesophyll tissue in leaves, at ten ppm cells become undifferentiated and at 50–600 μM concentration of Mo acquired alteration in the plant morphology, physiology especially dumb photosynthetic activity (Baldisserotto et al., 2013). Mo in backlog is a major gene in reducing plant growth and yield in poorly drained acidic soil, which is a suitable condition for the Mo availability (Rout and Das, 2002). Datta et al. (2011) reported that; in Cicer arietinum Mo concentration more than 7.v ppm reduced the root and shoot length and at concentration more than 1.v ppm altered the plant beefcake. Similarly Kumchai et al. (2013) illustrated his written report in the context of high level Mo (x mM) exposed to cabbage (Brassica oleracea), they reported the outcome of study that Mo decreased root and hypocotyls length and cotyledon length and too width (Datta et al., 2011). Izosimova (2005) reported the concentration of Ni (200–26,000 mg/kg) in contaminated soil, in comparison to optimum level Ni concentration (x–1000 mg/kg) in natural soil (Table 2; Figure 1). As stated by Rahman et al. (2005) Nitwo+ at elevated level leads to numerous toxicities (chlorosis and necrosis) and physiological modification in plant species. Furthermore, Pandey and Sharma (2002) elaborated that in plant species Nitwo+ provoke reduction of water content, this reduction is used to identify Ni2+ stress in plants. Theriault and Nkongolo (2016) reported nigh the Nickel and Copper Toxicity in White Birch (Betula papyrifera). According to the Warne et al. (2008) described that the increased concentration of Zn in soil hinders metabolic functions of plants that causes senescence and delayed growth. At increased concentration Zn create cytotoxic issue on plant growth and metabolism (Table ii; Figure one). It leads to major changes in the nucleolus of the root tips cells, cortical cells displayed disruption, and dilution of nuclear membrane at 7.5 mM dose of Zinc (Rout and Das, 2009). Similar results were obtained by Liu et al. (2016) in Solanum nigrum.

Tabular array 2. Effect of beneficial heavy metallic on unlike plants at high level.
Ameliorating Mechanism of Benificial Heavy Metal Toxicity
HMs such equally Co, Cu, Fe, Mn, Mo, Ni, and Zn are considered every bit beneficial elements that are required in modest concentration by the plants, their concentration at high level become toxic for plant at multiple level (Asati et al., 2016). Thus, researchers through different studies suggested the various mechanisms to ameliorate HMs toxicity. Zeid et al. (2013) ameliorated the cobalt toxicity from Medicago sativa by giving the pretreatment of the HMs solutions with atmospheric precipitation and EDTA (Ethylenediaminetetraacetic acrid) that reduced their retarding impact on growth and the metabolic activities. Li et al. (2008) through their experiment on copper stressed Arabidopsis thaliana alleviated the Cu toxicity past using silicon. Thus, Si (Silicon) decreased the leaf chlorosis, and enhanced root-shoot biomass. It too reduced the stress induced enzyme (phenylalanine ammonia-lyase). Si declined the RNA level of Arabidopsis copper transporter genes; copper transporter 1 (COPT1) and heavy metal ATPase subunit v (HMA5). Therefore, Si proved to better the plant resistance to Cu toxicity at multiple levels. Exposure of liming to Juglans regia, Robinia pseudoacacia, Eucalyptus sp., and Populus sp. plantations reported to alleviate Mn and Cu toxicity (Chatzistathis et al., 2015). Hajiboland et al. (2013) reported about the role of aluminum (300 μm) in reducing Fe toxicity in tea plant. Whereas, Dufey et al. (2014) reported the application of Si on rice establish to reduce the Fe generated toxicities. Rogalla and Römheld (2002) studied the toxic effects of Mn from low to loftier concentration (0·5–thousand μM) in Cucumis sativus supplied with Si as sodium silicate at ane.8 mM concentration, which reduced the generated stresses by decreasing Mn in intercellular washing fluid, mainly in the barium chloride (BaCl2) and DTPA (diethylenetriaminepentaacetic acrid)-exchangeable fraction of the leaf apoplast, in symplast surface area. Similar study was also reported by Maksimović et al. (2012) in Cucumber (Cucumis sativus). Si is also known to mitigate manganese mediated toxicities in plants (Liang et al., 2007). Kumchai et al. (2013) studied the role of proline to partially prevail over molybdenum induced stress in cabbage bulb. Institute hormone Gibberellic acrid has the potential to convalesce Ni induced stress; it has been proven by the Ali et al. (2015) in mungbeen plant. They further propounded that the application of gibberellic acid on mungbeen improved found growth and yield. Similarly, some other phytohormone jasmonic acrid was reported to amend institute growth parameters by reducing Ni mediated toxicity in Glycine max (Sirhindi et al., 2015). While Siddiqui et al. (2013) reported well-nigh the beneficiary role of salisylic acid and nitric oxide (NO) in mitigating Ni stress in wheat. Kaya et al. (2009) gave the exogenous application of Si (1.0 mM) in maize found grown in high zinc concentration that enhanced plant growth, chlorophyll content, and relative water content whereas reduced the membrane permeability and proline content. Another study was done by the researchers to alleviate Zn induced oxidative stress in radish (Raphanus sativus) seedling with the help of plant stress hormone 24-epibrassinolide, that reported to activated the antioxidative enzymatic arrangement (Ramakrishna and Rao, 2012).
Conclusion and Future Outlook
Soil serves the most important component accruing considerable corporeality of hazardous chemical pollutants from varying sources per yr. As well behaving as an oversized sink for chemic pollutants soil as well serves as a natural buffer past governing the overall transport of chemical substances to the environment. Plants reflect frequent sensitivity to both depression and loftier level concentration of heavy metals, at low level they serves as propitious elective for plant growth and development but on increasing its concentration beyond threshold limit it will imposes several inimical impacts in establish constituely thereby adversely influencing the soil fertility and development. Irksome but perpetual contamination of agricultural soil with heavy metal pollutant may significantly harm the environment and posess the major threats to public wellness and also build the major issues for subsequent discussion, as it gets accumulated in the soil and shows meaning accumulion in agricultural crops. According to the study of FAO (nutrient and agriculture organization of Un) (2009) globe population is increasing at a rapid rate and is predicted to reach nigh ix.vi billion till 2050. Therefore, the time to come global challenge is to mask the world'due south hunger through sustainable agronomics and food production. Some HMs such every bit Co, Cu, Iron, Mn, Mo, Ni, and Zn are considered as beneficial for plant growth and development. Plants require them in a limited quality. Whereas, at loftier level these metal ions tends to create differential level of toxicity in establish that in plough leads to inhibited plant growth, halt enzymatic and metabolic pathways and also create amercement to plant morphology and physiology that eventually reduced overall constitute productivity.
Therefore, based on the credible number of research reports information technology could be well-demarcated that only a required amount of heavy metal could revamp the physiological and morphological characteristics of plants. Thus, it will become essential to exaggerate the farther programmes for the improved comprehension of whole mechanism lying behind the synergistic and antagonistic action of heavy metals on plants to perpetuate the ecological harmony of the earth. Considering future perspectives, an efforts should exist fabricated to completely alleviate the exagregated level of essential metallic ions induced toxicity within the found tissue. Revealation of transportation mechanism at molecular level should also be made effective in context to plant beneficial HMs ion, also every bit reliability of one metal ion on the homeostasis of other metal ion. There is a need of much elaborated research on the machinery of metallic uptake and translocation in relation to their touch on on plant growth and development is required to keep pace with salubrious agricultural production.
Author Contributions
NA, VY, ShwS, RM, PA, SwS, and DT designed the manuscript, NA, VY, SwS, and DT wrote the manuscript. DT, NKD, PA, ShiS, and DC critically evaluated the manuscript.
Funding
Authors are thankful to the Academy Grants Committee, New Delhi for fiscal support. DT: Farther extends his thanks to the University Grants Committee for providing Dr. D. S. Kothari Mail-Doctoral Fellowship.
Conflict of Interest Statement
The authors declare that the enquiry was conducted in the absence of whatsoever commercial or fiscal relationships that could be construed as a potential conflict of interest.
Supplementary Cloth
The Supplementary Cloth for this article can be found online at: https://www.frontiersin.org/article/10.3389/fenvs.2016.00069/full#supplementary-material
References
Adamski, J. Grand., Danieloski, R., Deuner, S., Braga, Eastward. J., de Castro, L. A., and Peters, J. A. (2012). Responses to excess iron in sweet potato: impacts on growth, enzyme activities, mineral concentrations, and anatomy. Acta Physiol. Plant. 34, 1827–1836. doi: 10.1007/s11738-012-0981-three
CrossRef Total Text | Google Scholar
Akhtar, N., Sarker, K. A. M., Akhter, H., and Nothing, Grand. K. (2009). Outcome of planting time and micronutrient as zinc chloride on the growth, yield and oil content of Mentha piperita. People's republic of bangladesh J. Sci. Ind. Res. 44, 125–130. doi: ten.3329/bjsir.v44i1.2721
CrossRef Full Text | Google Scholar
Ali, Chiliad. A., Asghar, H. N., Khan, M. Y., Saleem, G., Naveed, Chiliad., and Niazi, N. Thou. (2015). Alleviation of nickel-induced stress in mungbean through application of gibberellic acid. Int. J. Agric. Biol. 17, 990–994. doi: 10.17957/IJAB/xv.0001
CrossRef Full Text | Google Scholar
Alloway, B. J. (2013). "Heavy metals and metalloids equally micronutrients for plants and animals," in Heavy Metals in Soils (Whiteknights: Springer), 195–209.
Anjum, N. A., Duarte, A. C., Pereira, E., and Ahmad, I. (2015). Plant-benign elements status cess in soil-plant system in the vicinity of a chemical manufacture complex: shedding light on forage grass safety issues. Environ. Sci. Pollut. R. 22, 2239–2246. doi: ten.1007/s11356-014-3478-three
PubMed Abstract | CrossRef Full Text | Google Scholar
Arora, A., Sairam, R. Yard., and Srivastava, G. C. (2002). Oxidative stress and antioxidative organization in plants. Curr. Sci. 82, 1227–1238.
Google Scholar
Asati, A., Pichhode, 1000., and Nikhil, K. (2016). Result of heavy metals on plants: an overview. Int. J. Appl. Innov. Eng. Manage. (IJAIEM). 5, 2319–4847.
Google Scholar
Azooz, One thousand. M., Abou-Elhamd, M. F., and Al-Fredan, M. A. (2012). Biphasic effect of copper on growth, proline, lipid peroxidation and antioxidant enzyme activities of wheat (Triticum aestivum cv. Hasaawi) at early growing stage. Aust. J. Crop Sci. 6, 688.
Google Scholar
Bakkaus, E., Gouget, B., Gallien, J. P., Khodja, H., Carrot, F., Morel, J. L., et al. (2005). Concentration and distribution of cobalt in college plants: the utilize of micro-PIXE spectroscopy. Nucl. Instrum. Methods Phys. Res. B 231, 350–356. doi: x.1016/j.nimb.2005.01.082
CrossRef Full Text | Google Scholar
Baldisserotto, C., Ferroni, Fifty., Pantaleoni, L., and Pancaldi, S. (2013). Comparison of photosynthesis recovery dynamics in floating leaves of Trapa natans afterwards inhibition past manganese or molybdenum: effects on photosystem 2. Plant Physiol. Biochem. 70, 387–395. doi: 10.1016/j.plaphy.2013.05.044
PubMed Abstract | CrossRef Full Text | Google Scholar
Barker, A. V., and Pilbeam, D. J. (eds.). (2015). Handbook of Plant Diet. Amherst, MA: CRC printing.
Google Scholar
Baxter, I., Muthukumar, B., Park, H. C., Buchner, P., Lahner, B., Danku, J., et al. (2008). Variation in molybdenum content across broadly distributed populations of Arabidopsis thaliana is controlled by a mitochondrial molybdenum transporter (MOT1). PLoS Genet. 4:e1000004. doi: x.1371/journal.pgen.1000004
PubMed Abstract | CrossRef Total Text | Google Scholar
Becana, 1000., Moran, J. F., and Iturbe-Ormaetxe, I. (1998). Iron-dependent oxygen free radical generation in plants subjected to ecology stress: toxicity and antioxidant protection. Establish Soil. 201, 137–147. doi: 10.1023/A:1004375732137
CrossRef Full Text | Google Scholar
Becher, Chiliad., Talke, I. N., Krall, L., and Krämer, U. (2004). Cross-species microarray transcript profiling reveals loftier constitutive expression of metal homeostasis genes in shoots of the zinc hyperaccumulator Arabidopsis halleri. Plant J. 37, 251–268. doi: 10.1046/j.1365-313X.2003.01959.ten
PubMed Abstract | CrossRef Total Text | Google Scholar
Bing, H., Wu, Y., Sun, Z., and Yao, Due south. (2011). Historical trends of heavy metal contamination and their sources in lacustrine sediment from Xijiu Lake, Taihu Lake Catchment, China. J. Environ. Sci. 23, 1671–1678. doi: 10.1016/S1001-0742(ten)60593-1
PubMed Abstract | CrossRef Full Text | Google Scholar
Blaylock, Chiliad. J., and Huang, J. W. (2000). "Phytoextraction of metals," in Phytoremediation of Toxic Metals: Using Plants to Clean Up the Environment, 53–70.
Chabukdhara, M., Munjal, A., Nema, A. K., Gupta, South. K., and Kaushal, R. K. (2016). Heavy metallic contamination in vegetables grown around peri-urban and urban-industrial clusters in Ghaziabad, Republic of india. Hum. Ecol. Adventure Assess. 22, 736–752. doi: 10.1080/10807039.2015.1105723
CrossRef Full Text | Google Scholar
Chatterjee, C., Gopal, R., and Dube, B. Thou. (2006). Impact of iron stress on biomass, yield, metabolism and quality of tater (Solanum tuberosum Fifty.). Sci. Hortic. 108, 1–6. doi: ten.1016/j.scienta.2006.01.004
CrossRef Full Text | Google Scholar
Chatzistathis, T., Alifragis, D., and Papaioannou, A. (2015). The influence of liming on soil chemical backdrop and on the consolation of manganese and copper toxicity in Juglans regia, Robinia pseudoacacia, Eucalyptus sp. and Populus sp. plantations. J. Environ. Manage. 150, 149–156. doi: x.1016/j.jenvman.2014.11.020
PubMed Abstract | CrossRef Full Text | Google Scholar
Chen, Z., Yan, W., Sun, Fifty., Tian, J., and Liao, H. (2016). Proteomic analysis reveals growth inhibition of soybean roots by manganese toxicity is associated with alteration of cell wall structure and lignification. J. Proteomics. 143, 151–160. doi: ten.1016/j.jprot.2016.03.037
PubMed Abstract | CrossRef Total Text | Google Scholar
Chopra, A. K., Pathak, C., and Parasad, G. (2009). Scenario of heavy metal contamination in agronomical soil and its management. J. Appl. Nat. Sci. ane, 99–108.
Google Scholar
Colangelo, E. P., and Guerinot, M. 50. (2006). Put the metallic to the petal: metal uptake and transport throughout plants. Curr. Opin. Plant Biol. 9, 322–330. doi: ten.1016/j.pbi.2006.03.015
PubMed Abstract | CrossRef Total Text | Google Scholar
Curie, C., Alonso, J. One thousand., Marie, L. E., Ecker, J. R., and Briat, J. F. (2000). Interest of NRAMP1 from Arabidopsis thaliana in iron transport. Biochem. J. 347, 749–755. doi: x.1042/bj3470749
PubMed Abstract | CrossRef Total Text | Google Scholar
Datta, J. Grand., Kundu, A., Hossein, S. D., Banerjee, A., and Mondal, N. 1000. (2011). Studies on the Impact of Micronutrient (Molybdenum) on germination, seedling growth and physiology of bengal grain (Cicer arietinum) under laboratory condition. Asian J. Crop Sci. 3, 55–67. doi: 10.3923/ajcs.2011.55.67
CrossRef Full Text | Google Scholar
De Dorlodot, South., Lutts, S., and Bertin, P. (2005). Effects of ferrous fe toxicity on the growth and mineral composition of an interspecific rice. J. Constitute Nutr. 28, ane–twenty. doi: 10.1081/PLN-200042144
CrossRef Full Text | Google Scholar
Demirevska-Kepova, K., Simova-Stoilova, L., Stoyanova, Z., Hölzer, R., and Feller, U. (2004). Biochemical changes in barley plants after excessive supply of copper and manganese. Environ. Exp. Bot. 52, 253–266. doi: ten.1016/j.envexpbot.2004.02.004
CrossRef Full Text | Google Scholar
Deng, West., Li, 10., An, Z., and Yang, L. (2016). The occurrence and sources of heavy metal contamination in peri-urban and smelting contaminated sites in Baoji, Communist china. Environ. Monit. Assess. 188, 1–viii. doi: 10.1007/s10661-016-5246-y
PubMed Abstract | CrossRef Full Text | Google Scholar
de Oliveira Jucoski, G., Cambraia, J., Ribeiro, C., de Oliveira, J. A., de Paula, Southward. O., and Oliva, M. A. (2013). Impact of iron toxicity on oxidative metabolism in young Eugenia uniflora L. plants. Acta Physiol. Institute. 35, 1645–1657. doi: ten.1007/s11738-012-1207-four
CrossRef Total Text | Google Scholar
Dey, S., Mazumder, P. B., and Paul, Southward. B. (2015). Copper-induced changes in growth and antioxidative mechanisms of tea plant (Camellia sinensis (Fifty.) O. Kuntze). Afr. J. Biotechnol. 14, 582–592. doi: x.5897/AJB2014.14279
CrossRef Full Text | Google Scholar
Ducic, T., and Polle, A. (2005). Ship and detoxification of manganese and copper in plants. Braz. J. Plant Physiol. 17, 103–112. doi: 10.1590/S1677-04202005000100009
CrossRef Total Text | Google Scholar
Dufey, I., Gheysens, Southward., Ingabire, A., Lutts, S., and Bertin, P. (2014). Silicon application in cultivated rices (Oryza sativa L and Oryza glaberrima Steud) alleviates iron toxicity symptoms through the reduction in iron concentration in the leaf tissue. J. Agron. Crop Sci. 200, 132–142. doi: 10.1111/jac.12046
CrossRef Total Text | Google Scholar
Ebru, O. One thousand. (2014). Nickel and Cobalt Furnishings on Maize Formation. Canakkale.
Frassinetti, S., Bronzetti, Chiliad. 50., Caltavuturo, L., Cini, G., and Della Croce, C. (2006). The office of zinc in life: a review. J. Environ. Pathol. Toxicol. Oncol. 25, 597–610. doi: 10.1615/JEnvironPatholToxicolOncol.v25.i3.40
PubMed Abstract | CrossRef Total Text | Google Scholar
Gad, North. (2012). Role and importance of cobalt nutrition on groundnut (Arachis hypogaea) product. World Appl. Sci. J. 20, 359–367. doi: ten.5829/idosi.wasj.2012.20.03.2819
CrossRef Full Text | Google Scholar
Gad, Due north., and Hassan, N. Chiliad. (2013). Office of cobalt and organic fertilizers amendments on tomato plant production in the newly reclaimed soil. World Appl. Sci. J. 22, 1527–1533. doi: 10.5829/idosi.wasj.2013.22.x.27413
CrossRef Total Text | Google Scholar
Gad, N., Mohammed, A. Chiliad., and Bekbayeva, L. K. (2013). Response of cowpea (Vigna anguiculata) to cobalt nutrition. Middle Due east J. Sci. Res. 14, 177–184. doi: 10.5829/idosi.mejsr.2013.14.2.2008
CrossRef Total Text | Google Scholar
Ghasemi, F., Heidari, R., Jameii, R., and Purakbar, L. (2012). Effects of Ni2+ toxicity on Hill reaction and membrane functionality in maize. J. Stress Physiol. Biochem. 8, 55–61.
Google Scholar
Gill, Thou. (2014). Heavy metal stress in plants: a review. Int. J. Adv. Res. 6, 1043–1055.
Google Scholar
Gill, Due south. S., and Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Found Physiol. Biochem. 48, 909–930. doi: 10.1016/j.plaphy.2010.08.016
PubMed Abstract | CrossRef Full Text | Google Scholar
Gopal, R., Sharma, Y. K., and Shukla, A. K. (2015). Outcome of molybdenum stress on growth, yield and seed quality in black gram. J. Plant Nutr. 39, 463–469. doi: ten.1080/01904167.2015.1016176
CrossRef Total Text | Google Scholar
Gupta, North., Ram, H., and Kumar, B. (2016). Mechanism of Zinc assimilation in plants: uptake, transport, translocation and accumulation. Rev. Environ. Sci. Biotechnol. 15, 89–109. doi: 10.1007/s11157-016-9390-1
CrossRef Total Text | Google Scholar
Hajiboland, R., Barceló, J., Poschenrieder, C., and Tolrà, R. (2013). Amelioration of fe toxicity: a mechanism for aluminum-induced growth stimulation in tea plants. J. Inorg. Biochem. 128, 183–187. doi: 10.1016/j.jinorgbio.2013.07.007
PubMed Abstruse | CrossRef Full Text | Google Scholar
Hirayama, T., Kieber, J. J., Hirayama, N., Kogan, G., Guzman, P., Nourizadeh, Due south., et al. (1999). RESPONSIVE-TO-ANTAGONIST1, a Menkes/Wilson disease–related copper transporter, is required for ethylene signaling in Arabidopsis. Cell 97, 383–393. doi: ten.1016/S0092-8674(00)80747-3
PubMed Abstract | CrossRef Full Text | Google Scholar
Hossain, M. A., Piyatida, P., da Silva, J. A. T., and Fujita, M. (2012). Molecular mechanism of heavy metal toxicity and tolerance in plants: key role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metallic chelation. J. Bot. 2012:37. doi: x.1155/2012/872875
CrossRef Full Text | Google Scholar
Hussain, D., Haydon, M. J., Wang, Y., Wong, E., Sherson, S. Chiliad., Young, J., et al. (2004). P-type ATPase heavy metal transporters with roles in essential zinc homeostasis in Arabidopsis. Establish Cell 16, 1327–1339. doi: 10.1105/tpc.020487
PubMed Abstract | CrossRef Full Text | Google Scholar
Hwang, J. U., Song, W. Y., Hong, D., Ko, D., Yamaoka, Y., Jang, S., et al. (2016). Establish ABC transporters enable many unique aspects of a terrestrial plant'southward lifestyle. Mol. Constitute ix, 338–355. doi: x.1007/s00299-016-2001-3
PubMed Abstract | CrossRef Total Text | Google Scholar
Izosimova, A. (2005). Modelling the Interaction between Calcium and Nickel in the Soil-Plant System. Bundesforschungsanstalt für Landwirtschaft (Braunschweig: FAL).
Google Scholar
Jacobs, J. A., and Testa, S. M. (2005). "Overview of chromium (VI) in the surround: groundwork and history" in Chromium (VI) Handbook, eds J. A. Jacobs, J. Guertin, and C. Avakian (New York: CRC Press), 1–21.
Jaleel, C. A., Changxing, Z., Jayakumar, K., and Iqbal, M. (2008). Low concentration of cobalt increases growth, biochemical constituents, mineral status and yield in Zea mays. J. Sci. Res. one, 128–137. doi: ten.3329/jsr.v1i1.1226
CrossRef Full Text | Google Scholar
Jayakumar, K., Jaleel, C. A., and Azooz, M. M. (2008). Impact of cobalt on germination and bulb growth of Eleusine coracana L. and Oryza sativa 50. under hydroponic culture. Global J. Mol. Sci. 3, 18–20.
Google Scholar
Jayakumar, G., and Vijayarengan, P. (2006). Influence of cobalt on seed germination and seedling growth of Vigna mungo (L.) Hepper. Found Curvation. six, 681–682.
Kabir, Thou., Iqbal, Yard. Z., and Shafiq, M. (2009). Effects of lead on seedling growth of Thespesia populnea L. Adv. Environ. Biol. 184–191.
Google Scholar
Kaiser, B. N., Gridley, 1000. L., Brady, J. Due north., Phillips, T., and Tyerman, S. D. (2005). The role of molybdenum in agricultural constitute production. Ann. Bot. 96, 745–754. doi: x.1093/aob/mci226
PubMed Abstruse | CrossRef Full Text | Google Scholar
Karmous, I., Bellani, L. Yard., Chaoui, A., El Ferjani, E., and Muccifora, S. (2015). Effects of copper on reserve mobilization in embryo of Phaseolus vulgaris Fifty. Environ. Sci. Pollut. R. 22, 10159–10165. doi: 10.1007/s11356-015-4208-one
PubMed Abstruse | CrossRef Full Text | Google Scholar
Kaya, C., Tuna, A. L., Sonmez, O., Ince, F., and Higgs, D. (2009). Mitigation effects of silicon on maize plants grown at loftier zinc. J. Plant Nutr. 32, 1788–1798. doi: 10.1080/01904160903152624
CrossRef Total Text | Google Scholar
Khan, K. R., and Khan, One thousand. One thousand. (2010). Effect of varying concentration of nickel and cobalt on the plant growth and yield of chickpea. Aust. J. Bones Appl. Sci. 4, 1036–1046.
Google Scholar
Khan, Z. I., Hussain, A., Ashraf, M., and McDowell, L. R. (2006). Mineral status of soils and forages in southwestern Punjab-Pakistan: micro-minerals. Asian Aust. J. Anim. Sci. nineteen:1139. doi: 10.5713/ajas.2006.1139
CrossRef Full Text | Google Scholar
Kitao, Thousand., Lei, T. T., Nakamura, T., and Koike, T. (2001). Manganese toxicity every bit indicated by visible foliar symptoms of Japanese white birch (Betula platyphylla var. japonica). Environ. Pollut. 111, 89–94. doi: 10.1016/S0269-7491(99)00332-2
PubMed Abstract | CrossRef Full Text | Google Scholar
Kleiber, T., and Graje, K, Thousand. (2015). Tomato reaction on excessive manganese nutrition. Bulg. J. Agric. Sci. 21, 118–125.
Google Scholar
Korshunova, Y. O., Eide, D., Clark, W. G., Guerinot, M. L., and Pakrasi, H. B. (1999). The IRT1 protein from Arabidopsis thaliana is a metallic transporter with a wide substrate range. Plant Mol. Biol. 40, 37–44. doi: 10.1023/A:1026438615520
PubMed Abstruse | CrossRef Full Text | Google Scholar
Kováčik, J., Babula, P., Hedbavny, J., and Švec, P. (2014). Manganese-induced oxidative stress in two ontogenetic stages of chamomile and amelioration past nitric oxide. Found Sci. 215, one–ten. doi: 10.1016/j.plantsci.2013.x.015
PubMed Abstract | CrossRef Full Text | Google Scholar
Krzesłowska, M. (2011). The cell wall in institute cell response to trace metals: polysaccharide remodeling and its office in defense strategy. Acta Physiol. Institute. 33, 35–51. doi: ten.1007/s11738-010-0581-z
CrossRef Full Text | Google Scholar
Kumchai, J., Huang, J. Z., Lee, C. Y., Chen, F. C., and Chin, S. W. (2013). Proline partially overcomes backlog molybdenum toxicity in cabbage seedlings grown in vitro. Genet. Mol. Res. 12, 5589–5601. doi: 10.4238/2013.November.xviii.eight
PubMed Abstract | CrossRef Full Text
Lasat, M. Chiliad., Pence, N. South., Garvin, D. F., Ebbs, S. D., and Kochian, L. 5. (2000). Molecular physiology of zinc transport in the Zn hyperaccumulator Thlaspi caerulescens. J. Exp. Bot. 51, 71–79. doi: 10.1093/jexbot/51.342.71
PubMed Abstruse | CrossRef Total Text | Google Scholar
Lewis, South., Donkin, One thousand. E., and Depledge, One thousand. H. (2001). Hsp70 expression in Enteromorpha intestinalis (Chlorophyta) exposed to environmental stressors. Aquat. Toxicol. 51, 277–291. doi: x.1016/S0166-445X(00)00119-3
PubMed Abstract | CrossRef Total Text | Google Scholar
Li, H. F., Gray, C., Mico, C., Zhao, F. J., and McGrath, S. P. (2009). Phytotoxicity and bioavailability of cobalt to plants in a range of soils. Chemosphere 75, 979–986. doi: 10.1016/j.Chemosphere.2008.12.068
PubMed Abstract | CrossRef Total Text | Google Scholar
Li, J., Leisner, S. Thousand., and Frantz, J. (2008). Alleviation of copper toxicity in Arabidopsis thaliana by silicon addition to hydroponic solutions. J. Am. Soc. Hortic. Sci. 133, 670–677.
Google Scholar
Li, Q., Chen, Fifty. S., Jiang, H. 10., Tang, Northward., Yang, L. T., Lin, Z. H., et al. (2010). Effects of manganese-excess on COtwo assimilation, ribulose-i, 5-bisphosphate carboxylase/oxygenase, carbohydrates and photosynthetic electron transport of leaves, and antioxidant systems of leaves and roots in Citrus grandis seedlings. BMC Establish Biol. ten:42. doi: ten.1186/1471-2229-10-42
PubMed Abstract | CrossRef Full Text | Google Scholar
Li, S., Zhou, Ten., Huang, Y., Zhu, 50., Zhang, S., Zhao, Y., et al. (2013). Identification and characterization of the zinc-regulated transporters, iron-regulated transporter-like protein (ZIP) gene family unit in maize. BMC Plant Biol. xiii:114. doi: x.1186/1471-2229-13-114
PubMed Abstract | CrossRef Full Text | Google Scholar
Li, X., Yang, Y., Jia, Fifty., Chen, H., and Wei, X. (2013). Zinc-induced oxidative impairment, antioxidant enzyme response and proline metabolism in roots and leaves of wheat plants. Ecotox. Environ. Saf. 89, 150–157. doi: 10.1016/j.ecoenv.2012.11.025
PubMed Abstract | CrossRef Full Text | Google Scholar
Liang, Y., Lord's day, Due west., Zhu, Y. G., and Christie, P. (2007). Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: a review. Environ Pollut. 147, 422–428. doi: 10.1016/j.envpol.2006.06.008
PubMed Abstract | CrossRef Full Text | Google Scholar
Liu, J., Reid, R. J., and Smith, F. A. (2000). The mechanism of cobalt toxicity in mung beans. Physiol. Plant. 110, 104–110. doi: ten.1034/j.1399-3054.2000.110114.x
CrossRef Total Text | Google Scholar
Liu, X., Chen, J., Wang, G. H., Wang, W. H., Shen, Z. J., Luo, M. R., et al. (2016). Hydrogen sulfide alleviates zinc toxicity past reducing zinc uptake and regulating genes expression of antioxidative enzymes and metallothioneins in roots of the cadmium/zinc hyperaccumulator Solanum nigrum L. Plant Soil 400, 177–192. doi: 10.1007/s11104-015-2719-seven
CrossRef Full Text | Google Scholar
Loftleidir, H. (2005). Essential Trace Elements for Plants, Animals and Humans. Reykjavik.
Google Scholar
Lottermoser, B. (2010). Mine Wastes: Label, Handling And Ecology Impacts. Townsville, QLD: Springer Science and Concern Media.
Google Scholar
Mahmood, T., and Islam, K. R. (2006). Response of rice seedlings to copper toxicity and acidity. J. Plant Nutr. 29, 943–957. doi: 10.1080/01904160600651704
CrossRef Full Text | Google Scholar
Maksimović, J. D., Mojović, M., Maksimović, V., Römheld, 5., and Nikolic, M. (2012). Silicon ameliorates manganese toxicity in cucumber by decreasing hydroxyl radical accumulation in the leaf apoplast. J. Exp Bot. 63, 2411–2420. doi: 10.1093/jxb/err359
PubMed Abstract | CrossRef Total Text | Google Scholar
Emamverdian, A., Ding, Y., Mokhberdoran, F., and Xie, Y. (2015). Heavy metal stress and some mechanisms of plant defence response. Scientific World Periodical. 2015:18. doi: x.1155/2015/756120
PubMed Abstract | CrossRef Total Text | Google Scholar
Manivasagaperumal, R., Vijayarengan, P., Balamurugan, S., and Thiyagarajan, G. (2011). Consequence of copper on growth, dry matter yield and nutrient content of Vigna radiata (L.) Wilczek. J. Phytol. 3, 53–62.
Google Scholar
Marschner, H. (1995). "Functions of mineral nutrients: macronutrients," Mineral Nutrition of Higher Plants, 2nd Edn (New York, NY: Bookish Printing), 299–312.
Marschner, P., and Rengel, Z. (eds.). (2007). Nutrient Cycling in Terrestrial Ecosystems, Vol. 10. Stuttgart: Springer-Verlag.
Google Scholar
McCall, K. A., Huang, C. C., and Fierke, C. A. (2000). Function and mechanism of zinc metalloenzymes. J. Nutr. 130, 1437S–1446S.
PubMed Abstract
McGrath, Southward. P., Micó, C., Curdy, R., and Zhao, F. J. (2010). Predicting molybdenum toxicity to higher plants: Influence of soil properties. Environ. Pollut. 158, 3095–3102. doi: x.1016/j.envpol.2010.06.027
PubMed Abstruse | CrossRef Full Text | Google Scholar
Mendel, R. R., and Schwarz, G. (2011). Molybdenum cofactor biosynthesis in plants and humans. Coordin. Chem. Rev. 255, 1145–1158. doi: 10.1016/j.ccr.2011.01.054
CrossRef Total Text | Google Scholar
Millaleo, R., Reyes-Díaz, Yard., Ivanov, A. G., Mora, M. L., and Alberdi, M. (2010). Manganese as essential and toxic element for plants: transport, aggregating and resistance mechanisms. J. Soil Sci. Plant Nutr. 10, 470–481. doi: 10.4067/S0718-95162010000200008
CrossRef Full Text | Google Scholar
Mizuno, T., Usui, K., Horie, Chiliad., Nosaka, S., Mizuno, N., and Obata, H. (2005). Cloning of three Zilch/Nramp transporter genes from a Ni hyperaccumulator plant Thlaspi japonicum and their Ni two+-transport abilities. Plant Physiol. Biochem. 43, 793–801. doi: x.1016/j.plaphy.2005.07.006
CrossRef Total Text | Google Scholar
Møller, I. K., Jensen, P. E., and Hansson, A. (2007). Oxidative modifications to cellular components in plants. Annu. Rev. Institute Biol. 58, 459–481. doi: 10.1146/annurev.arplant.58.032806.103946
PubMed Abstract | CrossRef Total Text | Google Scholar
Mukhopadhyay, Grand., Das, A., Subba, P., Bantawa, P., Sarkar, B., Ghosh, P., et al. (2013). Structural, physiological, and biochemical profiling of tea plantlets under zinc stress. Biol. Plant. 57, 474–480. doi: 10.1007/s10535-012-0300-2
CrossRef Full Text | Google Scholar
Nagajyoti, P. C., Lee, K. D., and Sreekanth, T. Five. Thousand. (2010). Heavy metals, occurrence and toxicity for plants: a review. Environ. Chem. Lett. 8, 199–216. doi: 10.1007/s10311-010-0297-8
CrossRef Total Text | Google Scholar
Nautiyal, N., Singh, South., and Chatterjee, C. (2005). Seed reserves of chickpea in relation to molybdenum supply. J. Sci. Food Agric. 85, 860–864. doi: 10.1002/jsfa.1929
CrossRef Total Text | Google Scholar
Oves, M., Khan, S., Qari, H., Felemban, North., and Almeelbi, T. (2016). Heavy Metals: biological importance and detoxification strategies. J. Bioremed. Biodegrad. 7:334. doi: 10.4172/2155-6199.1000334
CrossRef Full Text | Google Scholar
Pandey, N., and Sharma, C. P. (2002). Effect of heavy metals Co2+, Ni2+ and Cd2+ on growth and metabolism of cabbage. Plant Sci. 163, 753–758. doi: ten.1016/S0168-9452(02)00210-8
CrossRef Total Text | Google Scholar
Parmar, N. G., and Chanda, South. (2005). Effects of mercury and chromium on peroxidase and IAA oxidase enzymes in the seedlings of Phaseolus vulgaris. Turk. J. Biol. 29, 15–21.
Google Scholar
Pence, North. S., Larsen, P. B., Ebbs, S. D., Letham, D. 50., Lasat, M. M., Garvin, D. F., et al. (2000). The molecular physiology of heavy metallic transport in the Zn/Cd hyperaccumulator Thlaspi caerulescens. Proc. Natl. Acad. Sci. U.S.A. 97, 4956–4960. doi: x.1073/pnas.97.ix.4956
PubMed Abstruse | CrossRef Full Text | Google Scholar
Peralta-Videa, J. R., Lopez, G. L., Narayan, M., Saupe, One thousand., and Gardea-Torresdey, J. (2009). The biochemistry of environmental heavy metal uptake by plants: implications for the food chain. Int. J. Biochem. Jail cell Biol. 41, 1665–1677. doi: 10.1016/j.biocel.2009.03.005
PubMed Abstract | CrossRef Total Text | Google Scholar
Pichhode, M., and Nikhil, Grand. (2015). Consequence of copper mining dust on the soil and vegetation in india: a critical review. Int. J. Mod. Sci. Eng. Technol. 2, 73–76.
Google Scholar
Puig, S., Andrés-Colás, N., García-Molina, A., and PeñArrubia, L. (2007). Copper and fe homeostasis in Arabidopsis: responses to metallic deficiencies, interactions and biotechnological applications. Plant Cell Environ. 30, 271–290. doi: 10.1111/j.1365-3040.2007.01642.x
PubMed Abstract | CrossRef Full Text | Google Scholar
Rahman, H., Sabreen, S., Alam, S., and Kawai, Southward. (2005). Furnishings of nickel on growth and limerick of metal micronutrients in barley plants grown in food solution. J. Plant Nutr. 28, 393–404. doi: x.1081/PLN-200049149
CrossRef Full Text | Google Scholar
Ramakrishna, B., and Rao, Due south. S. R. (2012). 24-Epibrassinolide alleviated zinc-induced oxidative stress in radish (Raphanus sativus L.) seedlings by enhancing antioxidative organization. Institute Growth Regul. 68, 249–259. doi: 10.1007/s10725-012-9713-3
CrossRef Full Text | Google Scholar
Rana, D. S., and Noman, H. M. (2016). Effect of zinc levels and zinc bio-fertilizer on the productivity, quality and zinc-utilise efficiency in groundnut (Arachis hypogaea) and their residual upshot on succeeding wheat (Triticum aestivum). Ecol. Perspect. 656.
Google Scholar
Reeves, R. D., and Baker, A. J. (2000). "Metal-accumulating plants," Phytoremediation of Toxic Metals: Using Plants to Clean Up the Environment (New York, NY: Wiley), 193–229.
PubMed Abstruse
Rengel, Z. (2007). "Cycling of micronutrients in terrestrial ecosystems," in Nutrient Cycling in Terrestrial Ecosystems, eds P. Marschner and Z. Rengel (Berlin; Heidelberg: Springer), 93–121.
Rogalla, H., and Römheld, Five. (2002). Role of leaf apoplast in silicon-mediated manganese tolerance of Cucumis sativus L. Plant Cell Environ. 25, 549–555. doi: 10.1046/j.1365-3040.2002.00835.x
CrossRef Full Text | Google Scholar
Rout, Thou. R., and Das, P. (2002). Rapid hydroponic screening for molybdenum tolerance in rice through morphological and biochemical analysis. Rost Vyroba 48, 505–512.
Google Scholar
Rout, Yard. R., and Das, P. (2009). "Effect of metal toxicity on institute growth and metabolism: I. Zinc," in Sustainable Agriculture (Nayapalli: Springer), 873–884.
Google Scholar
Rout, J. R., Ram, S. Southward., Das, R., Chakraborty, A., Sudarshan, Grand., and Sahoo, S. Fifty. (2013). Copper-stress induced alterations in poly peptide profile and antioxidant enzymes activities in the in vitro grown Withania somnifera L. Physiol. Mol. Biol. Plants nineteen, 353–361. doi: 10.1007/s12298-013-0167-5
PubMed Abstract | CrossRef Full Text | Google Scholar
Sancenón, V., Puig, Due south., Mira, H., Thiele, D. J., and Peñarrubia, L. (2003). Identification of a copper transporter family in Arabidopsis thaliana. Constitute Mol. Biol. 51, 577–587. doi: 10.1023/A:1022345507112
PubMed Abstruse | CrossRef Full Text | Google Scholar
Shenker, M., Plessner, O. E., and Tel-Or, E. (2004). Manganese nutrition furnishings on tomato growth, chlorophyll concentration, and superoxide dismutase activity. J. Plant Physiol. 161, 197–202. doi: x.1078/0176-1617-00931
PubMed Abstract | CrossRef Full Text | Google Scholar
Shukla, R. (2010). Nickel level and toxicity and metabolism of white potato. Int. J. Vegetable Sci. 16, 160–166. doi: x.1080/19315260903358174
CrossRef Full Text | Google Scholar
Siddiqui, M. H., Al-Whaibi, M. H., Ali, H. One thousand., Sakran, A. Grand., Basalah, M. O., and Al Khaishany, One thousand. Y. (2013). Mitigation of nickel stress by the exogenous application of salicylic acid and nitric oxide in wheat. Aust. J. Crop Sci. 7, 1780.
Google Scholar
Singh, A., Parihar, P., Singh, R., and Prasad, S. M. (2016). An assessment to show toxic nature of beneficial trace metals: as well much of skilful thing tin can be bad. Int. J. Curr. Multidisciplinary Stud. ii, 141–144.
Google Scholar
Sirhindi, G., Mir, K. A., Sharma, P., Gill, S. S., Kaur, H., and Mushtaq, R. (2015). Modulatory role of jasmonic acid on photosynthetic pigments, antioxidants and stress markers of Glycine max L. under nickel stress. Physiol. Mol. Biol. Plants 21, 559–565. doi: ten.1007/s12298-015-0320-4
PubMed Abstruse | CrossRef Full Text | Google Scholar
Southron, J. L., Basu, U., and Taylor, G. J. (2004). Complementation of Saccharomyces cerevisiae ccc2 mutant by a putative P1B-ATPase from Brassica napus supports a copper-transporting function. FEBS Lett. 566, 218–222. doi: x.1016/j.febslet.2004.04.035
PubMed Abstruse | CrossRef Full Text | Google Scholar
Tchounwou, P. B., Yedjou, C. G., Patlolla, A. K., and Sutton, D. J. (2012). Heavy metal toxicity and the environment. In Molecular, Clinical and Environmental Toxicology (Basel: Springer), 133–164. doi: 10.1007/978-three-7643-8340-4_6
PubMed Abstract | CrossRef Full Text
Theriault, Chiliad., and Nkongolo, K. (2016). Nickel and copper toxicity and plant response mechanisms in white birch (Betula papyrifera). Bull Environ. Contam. Toxicol. 97, 171–176. doi: 10.1007/s00128-016-1842-3
PubMed Abstract | CrossRef Total Text | Google Scholar
Thomine, S., and Lanquar, V. (2011). "Fe send and signaling in plants," in Transporters and Pumps in Plant Signaling, eds Grand. Geisler and Thou. Bemema (Berlin; Heidelberg: Springer), 99–131.
Vert, G., Grotz, N., Dédaldéchamp, F., Gaymard, F., Guerinot, M. L., Briat, J. F., et al. (2002). IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for constitute growth. Plant Jail cell 14, 1223–1233. doi: 10.1105/tpc.001388
PubMed Abstruse | CrossRef Full Text | Google Scholar
Wang, D., Pang, Y. 10., Wang, W. Q., Wan, C. Y., Hou, J. L., Yu, F. L., et al. (2013). Result of molybdenum on secondary metabolic procedure of glycyrrhizic acrid in Glycyrrhiza uralensis Fisch. Biochem. Syst. Ecol. 50, 93–100. doi: 10.1016/j.bse.2013.03.045
CrossRef Full Text | Google Scholar
Warne, M. S. J., Heemsbergen, D., Stevens, D., McLaughlin, Grand., Cozens, Grand., Whatmuff, M., et al. (2008). Modeling the toxicity of copper and zinc salts to wheat in 14 soils. Envirol. Toxicol. Chem. 27, 786–792. doi: 10.1897/07-294.1
PubMed Abstract | CrossRef Total Text | Google Scholar
Wu, Fifty. B. (2016). Genetic and Physiological Analyses of the Tolerance Mechanisms to Ferrous Fe Toxicity in Rice (Oryza sativa L.). Doctoral dissertation, Dissertation, Bonn, Rheinische Friedrich-Wilhelms-Universität Bonn.
Google Scholar
Wuana, R. A., and Okieimen, F. East. (2011). Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol. 2011:xx. doi: ten.5402/2011/402647
CrossRef Full Text | Google Scholar
Zeid, I. M., Ghazi, South. M., and Nabawy, D. Thou. (2013). Alleviation of Co and Cr toxic effects on alfalfa. Int. J. Agron. Plant Prod. iv, 984–993.
Google Scholar
Source: https://www.frontiersin.org/articles/217521
Posted by: morrowtheatione.blogspot.com


0 Response to "What Will Happen If You Put Copper In A Fast Plant"
Post a Comment